Transcranial Magnetic Stimulation
 |
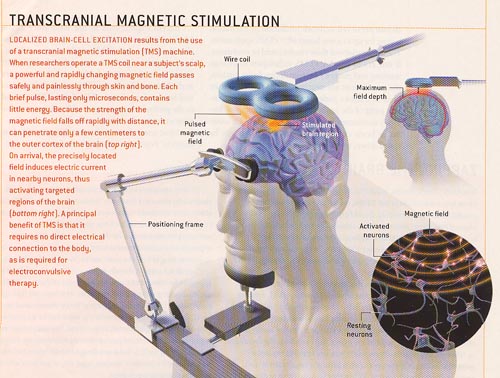 From article in Scientific American Sept 2003 From article in Scientific American Sept 2003 |
Biophysica’s portable repetitive Transcranial Magnetic Stimulator (TMS) is a form of Deep Brain Stimulation. TMS is a powerful highly effective non-invasive pulsed magnetic stimulator with a measurable range extending beyond 12 “(30 cm) and therefore capable of Deep Brain Stimulation. Biophysica’s TMS emits frequencies from 1Hz to 100Hz using our internal oscillator and a much wider range using the external pulse source (such as one of our frequency generators). This includes all the brain wave frequencies, especially Alpha and Theta Waves and 40Hz. Output waveform is biphasic (both positive and negative polarity). Pulse repetition frequency is adjustable by user and displayed on the front-panel frequency meter and should ideally be set between 1 and 5 Hz for maximum benefit. Range of effectiveness is more than sufficient to stimulate deep organs such as pituitary, pineal, pancreas, colon, bladder, lungs.
Two models are available:
Professional Clinic Model 1 The treatment applicator coil is mounted on an angled movable microphone arm and extends over the client sitting in a chair. This generator has Theta Burst, frequency and total pulse count display, external trigger input. Cost = $20,000.00 USD
Domestic Model 2 The treatment applicator coil is not rigidly mounted and is free to be moved while the client lies in a reclining position on a couch or bed. This flexibility allows the coil to be placed over feet, bones, joints, bowel, bladder. A case of bowel paralysis and another of endometriosis were cured with this technique. This generator outputs the same powerful energy of 3 Tesla and the user has complete control over the pulse frequency. Frequency display and Theta Burst are not needed in a domestic setting and not included. Cost = $15,000.00 USD
Our 7 minute TMS video by Dr Stewart can be seen
at http://www.youtube.com/watch?v=Xh-JP4dsRhY
Click here for our magnetic therapy page
Click here for price and ordering
TMS is used in many different areas of research and therapeutics including:
Price and Phone number at the bottom of the page are obsolete and should be 905-707-0500
TMS is used in many different areas of research and therapeutics including:
- Cognitive Neuroscience – in the investigation of learning, memory, creativity, speech, hearing, visual, perception and functional connection, cognitive enhancement.
- Psychiatry – to influence specific brain function within the dorsolateral prefrontal cortex To relieve anxiety, depression, bulemia (bulimia),schizophrenia and depersonalization as a better alternative to Electric Shock Treatmentalso called ECT.
- Neurophysiology – used in the stimulation of the peripheral and central nerve pathways. Eliciting motor evoked potentials to advanced brain mapping research techniques.
- Rehabilitation – used in the promotion of muscle recovery and the relief of pain and nerve spasticity.
- Effective, safer, less costly option to ECT and augmentation to antidepressants at http://www.psycom.net/tms.html
The following conditions have been successfully treated (some conditions need twice daily treatment) by Magnetic Deep Brain Stimulation:
Enhancing savant like creative abilities, Autism, auditory hallucinations, schizophrenia, anxiety, depression, memory impairment, sleepiness, depersonalization, mania, schizophrenia, Tinnitus, multiple sclerosis, Parkinsonism, post-stroke, Pain After Spinal Cord Injury, Panic Disorder, epilepsy, regional pain syndrome, migraine, PTSD,
Questions and Answers on Transcranial Magnetic Stimulation (TMS) from National Alliance on Mental Illness (NAMI) http://www.nami.org/Content/ContentGroups/Helpline1/Transcranial_Magnetic_Stimulation_(rTMS).htm
What is TMS?
Transcranial magnetic stimulation (TMS) is a technique for gently stimulating the brain. It utilizes a specialized electromagnet placed on the patient’s scalp that generates short magnetic pulses, roughly the strength of an MRI scanner’s magnetic field but much more focused. The magnetic pulses pass easily through the skull just like the MRI scanner fields do, but because they are short pulses and not a static field, they can stimulate the underlying cerebral cortex (brain). Low frequency (once per second) TMS has been shown to induce reductions in brain activation while stimulation at higher frequencies (> 5 pulses per second) has been shown to increase brain activation. It has also been shown that these changes can last for periods of time after stimulation is stopped. TMS was first developed in 1985, and has been studied significantly since 1995. TMS is not Vagus Nerve Stimulation or Acupuncture.
What disorders has TMS been shown to be useful for?
TMS is currently being investigated as a potential treatment for patients with major depression, patients who experience hallucinated “voices” and a variety of other psychiatric and neurological disorders. Over 1500 patients have been studied with TMS. For patients with major depression, many, but by no means all studies have shown clinical improvement following TMS. Recent studies that have used newer technology and stronger stimulation have shown much improved results. These pilot studies have taught researchers about how to better use TMS for depression.
For patients reporting auditory hallucinations (voices), research has not been as extensive but initial results have been promising and suggest that low frequency TMS administered to parts of the brain underlying speech perception may reduce these voices.
The Food and Drug Administration has not approved TMS for any psychiatric treatment at this time. Therefore TMS is only available as a research procedure. TMS has been approved in Canada and Israel as a treatment of depression for patients who have not responded to medications and who might ordinarily be considered for a trial of electroconvulsive therapy (ECT).
What does it feel like to receive TMS?
Generally TMS produces a slight knocking or tapping sensation on the head. This is also associated with a tapping sound produced by the TMS device. When administered at some stimulation sites it can cause contraction of the muscles of the scalp and occasionally the jaw. Mild headache and transient lightheadedness may sometimes result from TMS. These symptoms usually resolve by themselves shortly after the treatment is over.
Do you need to get anesthetized for TMS?
No. TMS is an outpatient procedure and does not require anesthesia or an IV. It can be administered in a physician’s office or clinic.
Does it hurt?
Approximately 5-10% of patients experience discomfort at the site of stimulation. In general this has not been a problem when administering TMS to patients volunteering for research studies.
How long does a treatment session last?
It depends on the research protocol, but generally each session takes about a half an hour. One procedure includes 2 daily sessions approximately 20 minutes each, with a 30-minute break between them. It is performed for 10 days, with a rest period of 2 days between the first 5 days and the second 5 days. There are 20 sessions in total.
How many times do you need to receive TMS?
Research protocols vary in the treatment duration, but most require at least two weeks of daily stimulation given five times per week, some require up to 6 weeks.
Are there any side-effects or risks associated with TMS?
Yes. The main risk of TMS, as with any pulsed energy, is inducing a seizure, though with close monitoring this complication has been very rare. No seizures have been reported in the scientific literature since safety guidelines have been implemented. For stimulation at low frequency (1 to 5 pulses per second) there have been no reports of seizures. Insofar as the brain is directly stimulated by TMS, there is a potential risk of disturbing the brain’s normal functions. However, in depression studies reported so far, no cognitive side-effects like loss of memory, negative changes in concentration and other cognitive capacities have been reported. This is in stark contract to the well known cognitive side effects associated with electroconvulsive therapy (ECT).
Is TMS widely available to patients in the U.S. and Canada?
TMS is not yet FDA cleared but has been approved in Canada and can be purchased from Biophysica.
Why are researchers evaluating TMS?
TMS has some very unique properties. It is non-invasive, (does not break the skin and can be delivered in a physician’s office) can easily be focused on small areas of the brain, and can change brain activity. This makes it particularly well suited for treating the brain, while minimizing side effects typical with other psychiatric treatments which affect areas of the brain and body not involved in the disorder.
Specifically for major depression, researchers understand there are a significant number of patients suffering from this disorder that are not helped by the available medications and other therapies, only receive partial benefit, or are not able to take medications at all. TMS offers hope that, if proven effective, many of these patients may be able to experience symptom relief.
Reviewed by Stanford Miller July 2004
Research on Transcranial Magnetic Stimulation
November 27 , 2007 Penn Research Shows Transcranial Magnetic Stimulation Effective in Treating Major Depression Non-drug, non-invasive treatment helps patients who have tried other options without success
PHILADELPHIA – Researchers at the University of Pennsylvania School of Medicine and other study sites have found that transcranial magnetic stimulation (TMS) – a non-invasive technique that excites neurons in the brain via magnetic pulses passed through the scalp – is a safe and effective, non-drug treatment with minimal side effects for patients with major depression who have tried other treatment options without benefit.
This study – the largest to-date studying TMS as a standalone treatment for major depression – appears in the December 1st issue of Biological Psychiatry.
TMS provides a well-tolerated treatment option to patients whose depression is otherwise treatment resistant,” says John P. O’Reardon, MD, Associate Professor of Psychiatry at Penn, and lead study author. “Since TMS is administered via the scalp and therefore goes directly to the brain, it allows the patient to avoid bodily side effects such as weight gain, sedation and/or sexual function.”
The study was conducted at 23 sites in the U.S., Australia, and Canada, and involved 301 medication-free patients with major depression who had not benefited from prior treatment. The patients were randomized to active or sham TMS for 4-6 weeks. Response and remission rates with active TMS were approximately twice those of sham. Additionally, there were no unexpected, serious side effects, and less than 5% of patients discontinued their TMS due to side effects. This is about three times better tolerated and safer than standard medications, which have about a 15% discontinuation rate due to side effects.
Dr. O’Reardon further comments, “As indicated by recent large scale, government-sponsored, studies of existing treatment options for major depression conducted by the National Institute of Health (the STAR-D reports), there is a great need to develop new, effective treatments for patients, especially those not benefiting from first line interventions. The results of this study indicate that TMS offers new hope to patients in this regard.”
Additional study authors are H. Brent Solvason, Philip G. Janiak, Shirlene Sampson, Keith E. Isenberg, Ziad Nahas, William M. McDonald, David Avery, Paul B. Fitzgerald, Colleen Loo, Mark A. Demitrack, Mark S. George, and Harold A. Sackeim.
Magnetic Stimulation Therapy Effective for Treatment-Resistant Depression: can beat depression when medication and therapy haven’t worked, according to the December issue of Mayo Clinic Health Letter. Dec 2009
The therapy, called transcranial magnetic stimulation (TMS), involves using brief powerful electromagnetic pulses to alter brain activity. The U.S. Food and Drug Administration (FDA) has approved the therapy for patients whose depression hasn’t improved with medications – estimated to be from 10 to 20 percent of those with the illness.
Patients treated with TMS may experience total remission of depression symptoms. A 50 percent improvement in depression symptoms is common.
A typical treatment schedule involves five, one-hour sessions a week for at least three to five weeks. During a session, the patient sits in a reclining chair while the magnetic coil is positioned and activated. Patients remain awake and alert as the coil alters brain activity. No anesthesia or invasive procedures are used. The benefits gradually emerge over several weeks.
A recent study compared TMS therapy in a group of people who had drug-resistant depression to a matched group of patients who received an inactive placebo form of TMS therapy. After four to six weeks, the TMS group was twice as likely to have remission of depression symptoms as the group receiving the placebo treatment.
While TMS is being used to treat depression at select medical centers, there are still many unknowns. Researchers don’t know how long the benefits might last. The general belief is that most patients who improve with TMS will continue to need some ongoing therapy for depression, whether it’s medication, counseling, additional TMS sessions or some combination of these therapies.
References and Links
- Stimulating brain with electricity aids learning speed , “applied to healthy adults: their speed of learning was also significantly increased”
- Electric current ‘boosts maths “targeting a part of the brain called the parietal lobe improved the ability of volunteers to solve numerical problems” 04 NOVEMBER 2010, HEALTH
- Magnets help regrow brain cells“Trans cranial magnetic stimulation (TMS) has been used to treat certain disorders, including depression and schizophrenia and to enhance memory and rehabilitate people after stroke”. 02 May 07 | Health
- Therapy hope for stroke victims Professor Rothwell said: “We can turn up or turn down the brain at will.” “It might be useful in epilepsy” 20 Jan 05 | Health
- Magnetic therapy helps stroke patients “The scientists are reawakening parts of the brain damaged through stroke” 07 SEPTEMBER 2011
- Magnetic therapy for spine injury “The therapy led to improved muscle and limb movement, and increased ability to feel sensations” 11 May 04 | Health
- Brain pulses stimulate deep sleep Enhances slow wave activity, believed to be critical to the restoration of mood and the ability to learn, think and remember, Edinburgh Sleep Centre 02 May 07 | Health
- Depression can be treated with electromagnets (better than drugs) September 25, 2010 by: David Gutierrez, http://www.naturalnews.com/029848_depression_electromagnets.html
- Magnetic brain therapy gets US green light at New Scientist http://www.newscientist.com/article/dn14998-magnetic-brain-therapy-gets-us-green-light.html “more than half of depressed patients showed an improvement in symptoms after receiving five 40-minute TMS sessions per week for four to six weeks”.
- Magnetic Stimulation Scores Modest Success as Antidepressant: Trial of Non-Invasive Treatment Used New, Convincing Sham Control from National Institute of Mental Health at http://www.nimh.nih.gov/science-news/2010/magnetic-stimulation-scores-modest-success-as-antidepressant.shtml
- “Man ‘roused from coma’ by a magnetic field” 15 October 2008 NewScientist.com news service at http://www.newscientist.com/article/mg20026783.400-man-roused-from-coma-by-a-magnetic-field.html
- “Enhancing savant like creative abilitieswith TMS” at http://query.nytimes.com/gst/fullpage.html?res=9506EFD81538F931A15755C0A9659C8B63
- “Autism and hyperactivity helped by transcranial magnetic stimulation” at University of Louisville, Kentucky Autism News by Matthew Hogg Monday, 14 April 2008 at http://www.ei-resource.org/news/autism-news/autism-helped-by-transcranial-magnetic-stimulation
- Maintenance Treatment With Transcranial Magnetic Stimulation in a Patient With Late-Onset Schizophrenia (hallucinations were greatly improved, by 80%) by EMMANUEL POULET, M.D., Ph.D., JEROME BRUNELIN, Ph.D., LASSAD KALLEL, M.D., THIERRY D’AMATO, M.D., Ph.D., and MOHAMED SAOUD, M.D., Ph.D., Lyon, France and Bron, France Am J Psychiatry 165:537-538, April, 2008 American Psychiatric Association
- “Portable device effective in zapping away migraine pain” Ohio State University Medical Center 26-Jun-2008 at http://www.eurekalert.org/pub_releases/2008-06/osum-pde062508.php
- Transcranial Magnetic Stimulator Claims To Zap Away Migraines, Submitted by News Accounton 25 June 2008 – 7:30am.Psychiatry . “Stimulation with magnetic pulses from the portable TMS device proved effective for the migraine patients” at http://www.scientificblogging.com/news_releases/transcranial_magnetic_stimulator_claims_to_zap_away_migraines
- Magnetic Brain Stimulation for Sleepiness, Memory Impairment, Anxiety Disorders and Schizophrenia (As the researchers wrote in the journal Cerebral Cortex, “TMS recipients were speedier in memory tests following the treatment” at Columbia University(funded by the Department of Defense).
- Magnetic Brain Stimulation Helps Depressed, Study Shows, Jan. 15, 2001 at Medical University of South Carolina.
- “Stimulating the Brain: Activating the brain’s circuitry with pulsed magnetic fields may help ease depression, enhance cognition, even fight fatigue”, Scientific American, September 2003 at sciam.com
- Literature search by psychiatrist Ivan Goldberg, MD at http://www.psycom.net/depression.central.transcranial.html
- Transcranial Magnetic Stimulation in the Treatment of Depression; A literature review by Ari A. Gershon, M.D., Pinhas N. Dannon, M.D., and Leon Grunhaus, M.D., Am J Psychiatry 160:835-845, May 2003 American Psychiatric Association at http://ajp.psychiatryonline.org/cgi/content/full/160/5/835
- Efficacy of TMS on Depression (with 53 references) in J. Psychiatry Neurosci 2005; 30(2) by Dr J. L. Couturier athttp://www.cma.ca/multimedia/staticContent/HTML/N0/l2/jpn/vol-30/issue-2/pdf/pg83.pdf
- Transcranial magnetic stimulation in psychiatry by Matthew Kirkcaldie and Saxby Pridmore Open Mind, the journal of the Tasmanian Association for Mental Health.
- Transcranial Magnetic Stimulation in Neuropsychiatry, edited by Mark S. George and Robert H. Belmaker. American Psychiatric Press, Washington DC 2000.
- Handbook of Transcranial Magnetic Stimulation, edited by Alvaro Pascual-Leone, Nick Davey, John Rothwell, Eric Wasserman, and Basant K. Puri. Oxford University Press, New York NY 2002.
- Educational Video on Transcranial Magnetic Stimulation (patient says “I feel like superman afterwards; better sleeping, appetite, energy, motivation, optimism”) from Fuqua Center For Late-Life Depression at Emory University at http://www.emoryhealthcare.org/departments/fuqua/patient_info/TMS.html
- Publications on treatment of “Depersonalization” using Transcranial Magnetic Stimulation from a clinical trial to evaluate the clinical efficacy of transcranial magnetic stimulation in the treatment of Depersonalization Disorder (DPD) Strong electromagnetic fields generated briefly (~1ms) but repetitively (1 to5 Hz) applied for 30mins, in five sessions per week for up to twelve weeks at http://clinicaltrials.gov/ct2/show/NCT00529217?cond=%22Depersonalization%22&rank=1
- Lisanby SH, Luber B, Schlaepfer TE, Sackeim HA: Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology 2003;28(10): 1852-65
- Lisanby SH, Kinnunen LH, Crupain MJ: Applications of TMS to therapy in psychiatry. J Clin Neurophysiol 2002;19(4): 344-60
- Lisanby SH: Update on magnetic seizure therapy: a novel form of convulsive therapy.. J ECT 2002;18(4): 182-8
- Blanke O, Mohr C, Michel CM, Pascual-Leone A, Brugger P, Seeck M, Landis T, Thut G. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. J Neurosci. 2005 Jan 19;25(3):550-7.
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997 May;48(5):1398-403.
- Hunter EC, Baker D, Phillips ML, Sierra M, David AS. Cognitive-behaviour therapy for depersonalisation disorder: an open study. Behav Res Ther. 2005 Sep;43(9):1121-30.
- Jimenez-Genchi AM. Repetitive transcranial magnetic stimulation improves depersonalization: a case report. CNS Spectr. 2004 May;9(5):375-6.
- Sierra M, Phillips ML, Ivin G, Krystal J, David AS. A placebo-controlled, cross-over trial of lamotrigine in depersonalization disorder. J Psychopharmacol. 2003 Mar;17(1):103-5.
- Simeon D, Guralnik O, Hazlett EA, Spiegel-Cohen J, Hollander E, Buchsbaum MS. Feeling unreal: a PET study of depersonalization disorder. Am J Psychiatry. 2000 Nov;157(11):1782-8.
- Simeon D, Guralnik O, Schmeidler J, Knutelska M. Fluoxetine therapy in depersonalisation disorder: randomised controlled trial. Br J Psychiatry. 2004 Jul;185:31-6.
- Simeon D, Knutelska M. An open trial of naltrexone in the treatment of depersonalization disorder. J Clin Psychopharmacol. 2005 Jun;25(3):267-70.
- Simeon D. Depersonalisation disorder: a contemporary overview. CNS Drugs. 2004;18(6):343-54. Review.
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998 Jan;108(1):1-16.
Selected references and free abstracts from over 2000 published articles on TMS at www.sciencedirect.com
Conditions treated auditory hallucinations, depression, schizophrenia, multiple sclerosis, Parkinsonism, post-stroke, Pain After Spinal Cord Injury, tinnitus, Panic Disorder, epilepsy, regional pain syndrome, migraine.
High-frequency oscillations change in parallel with short-interval intracortical inhibition after theta burst magnetic stimulation
Clinical Neurophysiology, Volume 119, Issue 2, February 2008, Pages 301-308
Takenobu Murakami, Kenji Sakuma, Takashi Nomura, Kenji Nakashima and Isao Hashimoto
| Abstract | Full Text + Links | PDF (331 K) |
Progress in treatment of auditory hallucinations with repetitive transcranial magnetic stimulation
Schizophrenia Research, Volume 98, Supplement 1, February 2008, Page 26
A. Aleman, I.E. Sommer, Z.J. Daskalakis and A. Vercammen
| Abstract | Full Text + Links | PDF (57 K) | |
Augmentative repetitive transcranial magnetic stimulation in treatment-resistant bipolar depressives
Schizophrenia Research, Volume 98, Supplement 1, February 2008, Pages 54-55
B. Dell’Osso, E. Mundo, N. D’Urso, S. Pozzoli and A.C. Altamura
| Abstract | Full Text + Links | PDF (72 K) | |
Repetitive transcranial magnetic stimulation efficiency in treatment-resistant auditory hallucinations
Schizophrenia Research, Volume 98, Supplement 1, February 2008, Page 56
S. Grenier, G. Fouldrin, G. Allio, G. Opolczynski and F. Thibaut
| Abstract | Full Text + Links | PDF (59 K) | |
The physiological basis of transcranial magnetic stimulation
Trends in Cognitive Sciences, In Press, Corrected Proof, Available online 1 February 2008
Sven Bestmann
| Abstract | Full Text + Links | PDF (250 K) | |
Repetitive transcranial magnetic stimulation (rTMS) and magnetic seizure therapy (MST)
Journal of Affective Disorders, In Press, Corrected Proof, Available online 30 January 2008
T.E. Schlaepfer, C. Frick and S. Kayser
| Abstract | Full Text + Links | PDF (95 K) | |
A study of the effectiveness of bilateral transcranial magnetic stimulation in the treatment of the negative symptoms of schizophrenia
Brain Stimulation, Volume 1, Issue 1, January 2008, Pages 27-32
Paul B. Fitzgerald, Sally Herring, Kate Hoy, Susan McQueen, Rebecca Segrave, Jayashri Kulkarni and Zafiris J. Daskalakis
| Abstract | Full Text + Links | PDF (156 K) |
Investigation of paroxysmal dystonia in a patient with multiple sclerosis: A transcranial magnetic stimulation study
Clinical Neurophysiology, Volume 119, Issue 1, January 2008, Pages 63-70
Carlo Trompetto, Laura Avanzino, Marco Bove, Alessandro Buccolieri, Antonio Uccelli and Giovanni Abbruzzese
| Abstract | Full Text + Links | PDF (628 K) | |
Enhancing language performance with non-invasive brain stimulation—A transcranial direct current stimulation study in healthy humans
Neuropsychologia, Volume 46, Issue 1, 2008, Pages 261-268
Roland Sparing, Manuel Dafotakis, Ingo G. Meister, Nivethida Thirugnanasambandam and Gereon R. Fink
| Abstract | Full Text + Links | PDF (457 K) |
Recovery of motor disability and spasticity in post-stroke after repetitive transcranial magnetic stimulation (rTMS)
Brain Research Bulletin, In Press, Uncorrected Proof, Available online 26 December 2007
J. Málly and E. Dinya
| Abstract | Full Text + Links | PDF (405 K) |
The clinical diagnostic utility of transcranial magnetic stimulation: Report of an IFCN committee
Clinical Neurophysiology, In Press, Corrected Proof, Available online 11 December 2007
Robert Chen, Didier Cros, Antonio Curra, Vincenzo Di Lazzaro, Jean-Pascal Lefaucheur, Michel R. Magistris, Kerry Mills, Kai M. Rösler, William J. Triggs, Yoshikazu Ugawa and Ulf Ziemann
| Abstract | Full Text + Links | PDF (838 K) |
The Effect of a Series of Repetitive Transcranial Magnetic Stimulations of the Motor Cortex on Central Pain After Spinal Cord Injury
Archives of Physical Medicine and Rehabilitation, Volume 88, Issue 12, December 2007, Pages 1574-1580
Ruth Defrin, Leon Grunhaus, Doron Zamir and Gabi Zeilig
| Abstract | Full Text + Links | PDF (160 K) |
Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial
Biological Psychiatry, Volume 62, Issue 11, 1 December 2007, Pages 1208-1216
John P. O’Reardon, H. Brent Solvason, Philip G. Janicak, Shirlene Sampson, Keith E. Isenberg, Ziad Nahas, William M. McDonald, David Avery, Paul B. Fitzgerald, Colleen Loo, Mark A. Demitrack, Mark S. George and Harold A. Sackeim
| Abstract | Full Text + Links | PDF (318 K) |
Modulation of cardiac autonomic functions in patients with major depression treated with repetitive transcranial magnetic stimulation
Journal of Affective Disorders, Volume 104, Issues 1-3, December 2007, Pages 231-236
Kaviraja Udupa, T.N. Sathyaprabha, Jagadisha Thirthalli, K.R. Kishore, T.R. Raju and B.N. Gangadhar
| Abstract | Full Text + Links | PDF (163 K) | |
Patterns of response to repetitive transcranial magnetic stimulation (rTMS) in major depression: Replication study in drug-free patients
Journal of Affective Disorders, In Press, Corrected Proof, Available online 26 October 2007
Eva-Lotta Brakemeier, Gregor Wilbertz, Silke Rodax, Heidi Danker-Hopfe, Bettina Zinka, Peter Zwanzger, Nicola Grossheinrich, Bálint Várkuti, Rainer Rupprecht, Malek Bajbouj and Frank Padberg
| Abstract | Full Text + Links | PDF (212 K) |
An open study of repetitive transcranial magnetic stimulation in treatment-resistant depression with Parkinson’s disease
Clinical Neurophysiology, Volume 118, Issue 10, October 2007, Pages 2189-2194
Charles M. Epstein, Marian L. Evatt, Agnes Funk, Lhys Girard-Siqueira, Nichole Lupei, Larisa Slaughter, Saima Athar, Joanne Green, William McDonald and Mahlon R. DeLong
| Abstract | Full Text + Links | PDF (140 K) |
P.2.g.004 Repetitive transcranial magnetic stimulation for depression: a randomised controlled trial with 4-month follow-up
European Neuropsychopharmacology, Volume 17, Supplement 4, October 2007, Pages S392-S393
D.M. McLoughlin, A. Mogg, G. Pluck, S. Eranti, S. Landau, R. Purvis, R.G. Brown, R. Howard, V. Curtis and M. Philpot
| PDF (70 K) | |
Which tinnitus patients benefit from transcranial magnetic stimulation?
Otolaryngology – Head and Neck Surgery, Volume 137, Issue 4, October 2007, Pages 589-595
Tobias Kleinjung, Thomas Steffens, Philipp Sand, Tobias Murthum, Goeran Hajak, Juergen Strutz, Berthold Langguth and Peter Eichhammer
| Abstract | Full Text + Links | PDF (632 K) |
Safety study of high-frequency transcranial magnetic stimulation in patients with chronic stroke
Clinical Neurophysiology, Volume 118, Issue 9, September 2007, Pages 2072-2075
M.P. Lomarev, D.Y. Kim, S. Pirio Richardson, B. Voller and M. Hallett
| Abstract | Full Text + Links | PDF (134 K) | |
Repetitive Transcranial Magnetic Stimulation (rTMS) in the treatment of Panic Disorder (PD) with comorbid major depression
Journal of Affective Disorders, Volume 102, Issues 1-3, September 2007, Pages 277-280
Antonio Mantovani, Sarah H. Lisanby, Fulvio Pieraccini, Monica Ulivelli, Paolo Castrogiovanni and Simone Rossi
| Abstract | Full Text + Links | PDF (109 K) |
Positive predictors for antidepressive response to prefrontal repetitive transcranial magnetic stimulation (rTMS)
Journal of Psychiatric Research, Volume 41, Issue 5, August 2007, Pages 395-403
Eva-Lotta Brakemeier, Alexander Luborzewski, Heidi Danker-Hopfe, Norbert Kathmann and Malek Bajbouj
| Abstract | Full Text + Links | PDF (283 K) | |
Transcranial Magnetic Stimulation in Veterans with Tinnitus
Otolaryngology – Head and Neck Surgery, Volume 137, Issue 2, Supplement 1, August 2007, Page P181
Scott L. Lee, Steven M. Silver and Anthony Cacace
| Abstract | Full Text + Links | PDF (34 K) | |
Low frequency repetitive transcranial magnetic stimulation over premotor cortex can improve cortical tremor
Clinical Neurophysiology, Volume 118, Issue 7, July 2007, Pages 1557-1562
E. Houdayer, H. Devanne, L. Tyvaert, L. Defebvre, P. Derambure and F. Cassim
| Abstract | Full Text + Links | PDF (188 K) |
Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature
Epilepsy & Behavior, Volume 10, Issue 4, June 2007, Pages 521-528
Erica Hyunji Bae, Lara M. Schrader, Katsuyuki Machii, Miguel Alonso-Alonso, James J. Riviello Jr., Alvaro Pascual-Leone and Alexander Rotenberg
| Abstract | Full Text + Links | PDF (160 K) | |
Repetitive transcranial magnetic stimulation over the motor cortex can change the pain perception in patients with complex regional pain syndrome
European Journal of Pain, Volume 11, Issue 1, Supplement 1, June 2007, Page 188
H. Picarelli, M.J. Teixeira, M.A. Marcolin, M.L. Myczkowski and T.B. Luvisotto
| Abstract | Full Text + Links | PDF (41 K) |
Repetitive Transcranial Magnetic Stimulation (rTMS) in Experimentally Induced and Chronic Neuropathic Pain: A Review
The Journal of Pain, Volume 8, Issue 6, June 2007, Pages 453-459
Raphael J. Leo and Tariq Latif
| Abstract | Full Text + Links | PDF (134 K) | |
